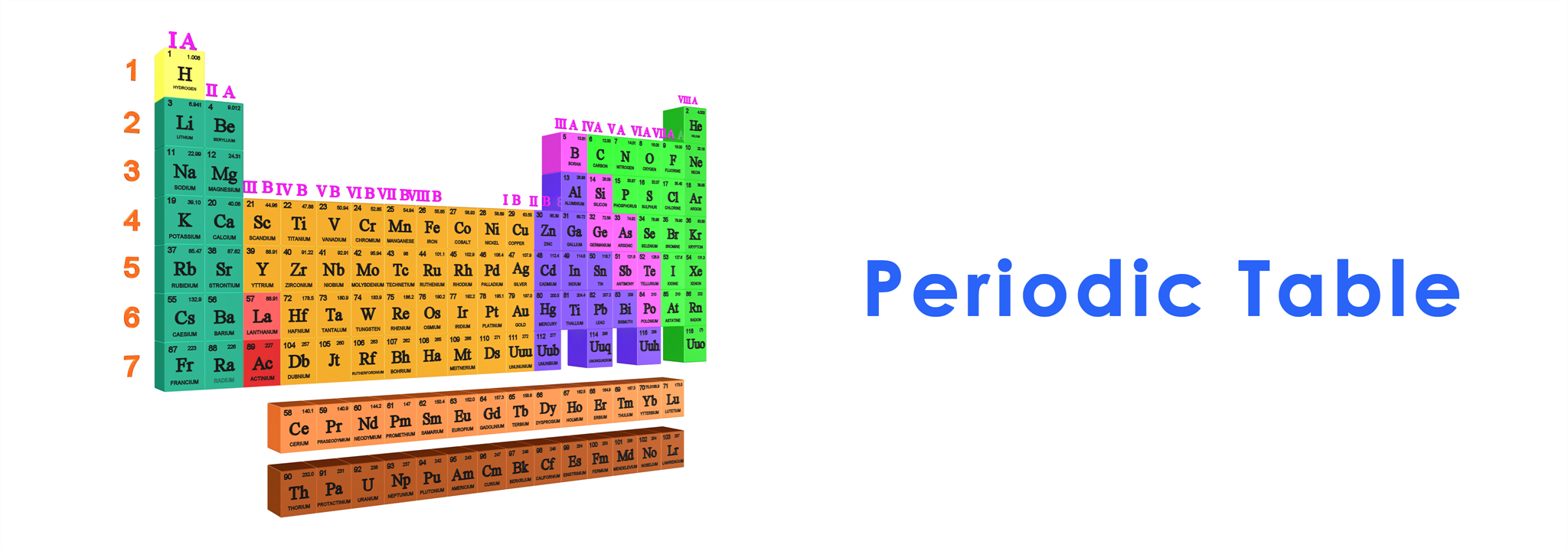
The periodic table is a tabular arrangement of chemical elements based on their atomic structure and similar properties, known as the "Periodic Law," for the purpose of facilitating use and study. The arrangement also allows for the explanation of relationships between various elemental properties, as well as predicting chemical properties and behaviors of yet-to-be-discovered or newly synthesized elements.
An element is the fundamental structure of matter, a pure substance that cannot be further divided by chemical means. Elements are composed of tiny particles called atoms, which are the basic building blocks of matter. Elements consist of atoms of the same type.
Each atom of an element consists of tiny fundamental particles: protons, which have a positive electrical charge; electrons, which have a negative electrical charge; and neutrons, which have no electrical charge. Elements are composed of combinations of these tiny particles, with the number of particles within the atom determining unique properties. The differences in properties of various elements in nature are thus defined by the number of particles within the atoms.

The periodic table was first studied, researched, and compiled by Russian chemist Dmitri Ivanovich Mendeleev in 1869. He arranged each element according to the structure within atoms. Mendeleev's table allowed for the prediction of elements or properties of elements yet to be discovered. For example, Gallium was predicted by Mendeleev in 1871 before its discovery in 1875.
At that time, the periodic table was widely accepted and published, but it included only 69 known elements before new elements were discovered later, leading to further development and improvement of the table. Currently, there are up to 119 elements, from Hydrogen (element 1) to Oganesson (element 118).
Of all 98 natural elements, the remaining 16 elements, from Iridium (element 99) to Oganesson (element 118), have been synthesized only in laboratory settings. Among all 98 natural elements, 84 are stable, while the remaining 14 are radioactive and decay into the former. No element heavier than Iridium (element 99) has been found in a pure elemental state in quantities visible to the naked eye.
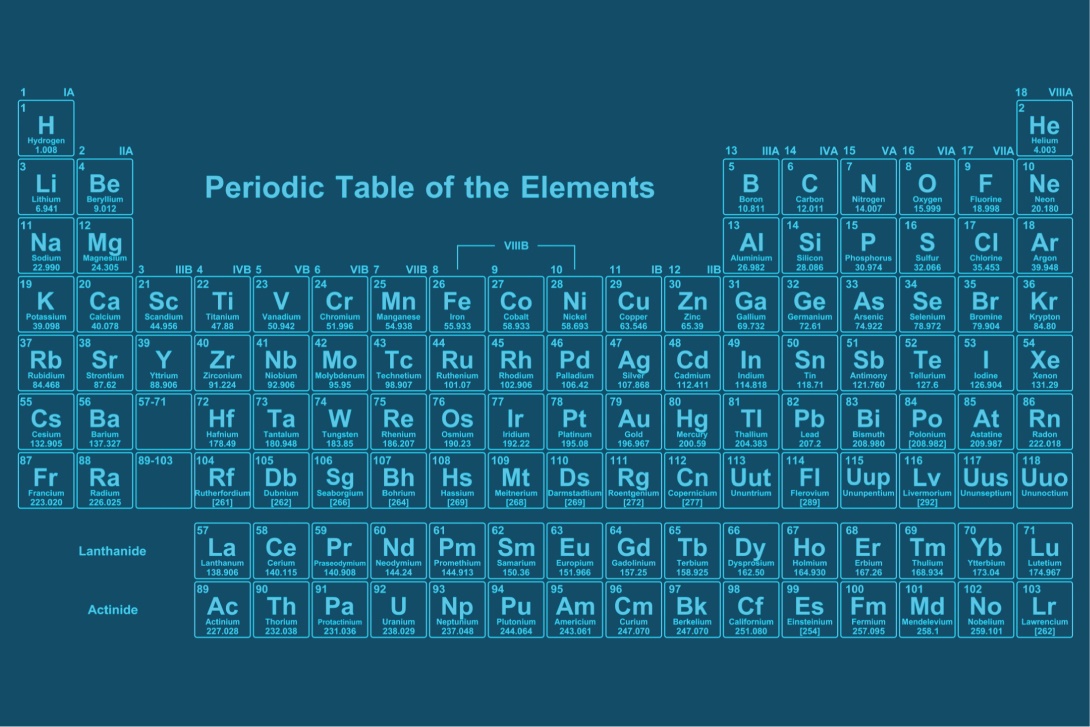
In the standard periodic table, elements are arranged from left to right and top to bottom based on the number of protons in the nucleus of the atom, or atomic number. The modern periodic table divides all elements into 18 groups vertically, where elements with similar properties are placed in the same group based on their valence electrons, or the number of electrons in the outermost shell. Each group in the periodic table is designated by a Roman numeral or an Arabic numeral, such as IA or 1A.
Moreover, elements in some groups also have specific names. For example, Group IA elements are called "Alkali Metals," Group IIA elements are called "Alkaline Earth Metals," Group VIIA elements are called "Halogens," and Group VIIIA elements are commonly referred to as "Noble Gases."
Additionally, there are 7 periods horizontally indicating the number of electron shells. The number of electrons and protons of elements in the same period increases by one as you move from left to right across the table, while the metallic properties decrease from left to right. Meanwhile, electrons are arranged in new shells when the previous shell is filled, indicating the beginning of a new period in the periodic table.
This arrangement results in the repetition of elements with similar chemical properties as the atomic number increases. Furthermore, as the mass increases, most elements become less stable, making those with higher atomic numbers less likely to occur naturally.
The periodic table also categorizes elements into groups based on metallic properties. There are three main groups: